Atrial Fibrillation Clinical Trials & Pipeline Overview: Insights into 12+ Leading Companies and 15+ Novel Treatments | DelveInsight
Atrial Fibrillation, the most common cardiac arrhythmia, poses serious risks such as stroke and heart failure. With its prevalence rising sharply among older adults, the aging global population is expected to be a major driver of market growth for AF therapies.
New York, USA, Sept. 30, 2025 (GLOBE NEWSWIRE) -- Atrial Fibrillation Clinical Trials & Pipeline Overview: Insights into 12+ Leading Companies and 15+ Novel Treatments | DelveInsight
Atrial Fibrillation, the most common cardiac arrhythmia, poses serious risks such as stroke and heart failure. With its prevalence rising sharply among older adults, the aging global population is expected to be a major driver of market growth for AF therapies.
DelveInsight’s 'Atrial Fibrillation Pipeline Insight 2025' report provides comprehensive global coverage of pipeline atrial fibrillation therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the atrial fibrillation pipeline domain.
Key Takeaways from the Atrial Fibrillation Pipeline Report
- DelveInsight’s atrial fibrillation pipeline report depicts a robust space with 12+ active players working to develop 15+ pipeline atrial fibrillation drugs.
- Key atrial fibrillation companies such as Novartis AG, HUYA Bioscience, Milestone Pharmaceuticals, Verseon, Thryv Therapeutics Inc., Acesion Pharma, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and others are evaluating new atrial fibrillation drugs to improve the treatment landscape.
- Promising pipeline atrial fibrillation therapies, such as Abelacimab, HBI-3000, Etripamil, VE 1902, THRV-1268, AP31969, BAY 3670549, Milvexian, and others, are in different phases of atrial fibrillation clinical trials.
- In September 2025, Thryv Therapeutics Inc. announced the clearance of its Investigational New Drug (IND) application for THRV-1268 by the US Food and Drug Administration (FDA) to initiate its clinical program in heart failure (HF) and atrial fibrillation.
- In August 2025, Thryv Therapeutics Inc. announced that new preclinical results on its lead compound, THRV-1268 will be presented at the upcoming European Society of Cardiology (ESC)Search organization Congress 2025, taking place August 29 – September 1, 2025, in Madrid, Spain.
- In May 2025, Bayer announced the initiation of a Phase I clinical trial with BAY 3670549, an investigational highly selective G-protein-coupled inwardly rectifying potassium channel 4 (GIRK4) inhibitor, which has the potential to help control the electrical activity of heart cells in patients with atrial fibrillation (AFib).
- In April 2025, HUYABIO International announced the presentation of new patient data for HBI-3000. The company presents positive clinical results from the HBI-3000 Phase IIA clinical trial of novel multi-ion channel blocker for acute atrial fibrillation
- In February 2025, Novartis announced that it had entered into an agreement to acquire Anthos Therapeutics, Inc., a Boston-based, privately held, clinical-stage biopharmaceutical company with abelacimab, a late-stage medicine in development for the treatment of and systemic embolism in patients with atrial fibrillation.
Request a sample and discover the recent advances in atrial fibrillation drugs @ Atrial Fibrillation Pipeline Report
The atrial fibrillation pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage atrial fibrillation drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the atrial fibrillation clinical trial landscape.
Atrial Fibrillation Overview
Atrial fibrillation (AFib) is the most common form of heart rhythm disorder, in which the heartbeat becomes irregular and often rapid. It happens due to abnormal electrical activity in the atria, the upper chambers of the heart, causing them to beat ineffectively. This disrupts smooth blood flow and can cause blood clots, increasing the risk of stroke and other heart-related complications. AFib can appear occasionally, last longer than a week (persistent), or become a long-term condition. Symptoms vary widely in severity.
Typical signs include a racing or fluttering heart, tiredness, dizziness, shortness of breath, and chest discomfort. However, some individuals may not feel any symptoms, a condition known as silent AFib, which is still dangerous. Symptom intensity can vary based on heart rate, existing heart conditions, and overall health.
A variety of factors can lead to AFib. These include high blood pressure, coronary artery disease, heart failure, valve disorders, and congenital heart abnormalities. Other non-heart-related triggers include overactive thyroid, obesity, diabetes, sleep apnea, and excessive alcohol use. The risk also increases with age. Family history can also contribute, suggesting a genetic link.
In AFib, the heart’s normal electrical rhythm becomes disorganized, often due to abnormal electrical signals arising from areas like the pulmonary veins. These signals cause the atria to quiver instead of contracting properly, leading to an irregular heartbeat. Over time, structural and electrical changes in the atria may occur, making AFib more persistent and harder to treat. Diagnosis is usually made through an electrocardiogram (ECG), with further tests such as Holter monitoring, echocardiography, and blood work to evaluate heart function and identify causes.
Treatment aims to relieve symptoms, prevent complications like stroke, and correct underlying conditions. Common therapies include medications such as beta-blockers, calcium channel blockers, and anticoagulants, or electrical cardioversion to restore normal rhythm. In more complex cases, procedures like catheter ablation or surgery are used to eliminate the faulty electrical areas. Lifestyle changes, such as keeping a healthy weight, controlling blood pressure, and limiting alcohol, are also important parts of managing AFib.
Find out more about atrial fibrillation drugs @ Atrial Fibrillation Treatment
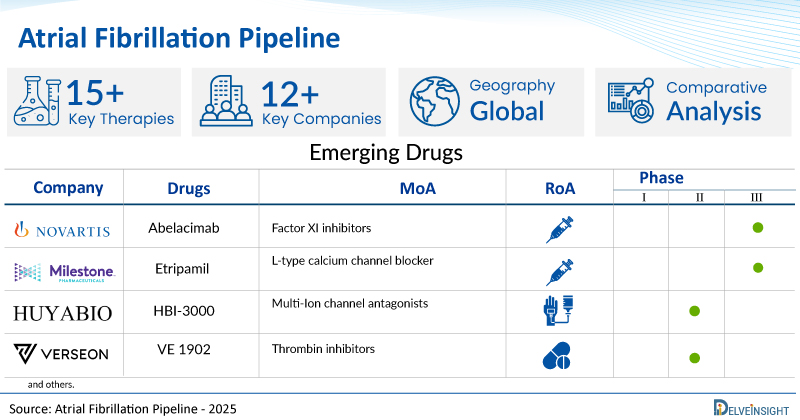
A snapshot of the Pipeline Atrial Fibrillation Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Abelacimab | Novartis AG | III | Factor XI inhibitors | Subcutaneous |
| Etripamil | Milestone Pharmaceuticals Inc. | III | L-type calcium channel blocker | Intranasal |
| HBI-3000 | HUYA Bioscience | II | Multi-Ion channel antagonists | Intravenous |
| VE 1902 | Verseon | I | Thrombin inhibitors | Oral |
| THRV-1268 | Thryv Therapeutics Inc. | I | SGK1 inhibitor | Oral |
| AP31969 | Acesion Pharma | I | SK ion channel inhibitor | Oral |
Learn more about the emerging atrial fibrillation therapies @ Atrial Fibrillation Clinical Trials
Atrial Fibrillation Therapeutics Assessment
The atrial fibrillation pipeline report proffers an integral view of the emerging atrial fibrillation therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Atrial Fibrillation Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: Factor XI inhibitors, Multi-Ion channel antagonists, SGK1 inhibitor, L-type calcium channel blocker, SK ion channel inhibitor, Thrombin inhibitors
- Key Atrial Fibrillation Companies: Novartis AG, HUYA Bioscience, Milestone Pharmaceuticals, Verseon, Thryv Therapeutics Inc., Acesion Pharma, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and others.
- Key Atrial Fibrillation Pipeline Therapies: Abelacimab, HBI-3000, Etripamil, VE 1902, THRV-1268, AP31969, BAY 3670549, Milvexian, and others.
Dive deep into rich insights for new atrial fibrillation treatments, visit @ Atrial Fibrillation Drugs
Table of Contents
| 1. | Atrial Fibrillation Pipeline Report Introduction |
| 2. | Atrial Fibrillation Pipeline Report Executive Summary |
| 3. | Atrial Fibrillation Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Atrial Fibrillation Clinical Trial Therapeutics |
| 6. | Atrial Fibrillation Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Atrial Fibrillation Pipeline: Late-Stage Products (Phase III) |
| 8. | Atrial Fibrillation Pipeline: Mid-Stage Products (Phase II) |
| 9. | Atrial Fibrillation Pipeline: Early-Stage Products (Phase I) |
| 10. | Atrial Fibrillation Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Atrial Fibrillation Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Atrial Fibrillation Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the atrial fibrillation pipeline therapeutics, reach out @ Atrial Fibrillation Therapeutics
Related Reports
Atrial Fibrillation Epidemiology Forecast
Atrial Fibrillation Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted atrial fibrillation epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Atrial Fibrillation Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atrial fibrillation companies, including HUYA Bioscience, InCarda Therapeutics, Inc., Milestone Pharmaceuticals, Verseon, Thryv Therapeutics Inc., Vivasc Therapeutics, among others.
Heart Failure Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key heart failure companies, including Mesoblast, Rivus Pharmaceuticals, Heartseed Inc., StemCardia, Eli Lilly and Company, BioCardia, Bristol Myers Squibb, Sardocor Corp., AstraZeneca, Tenaya Therapeutics, Salubris Bio Therapeutics, Moderna Therapeutics, Cytokinetics, Eli Lilly and Company, Applied Therapeutics, Help Therapeutics, Stealth BioTherapeutics, Actelion Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Merck & Co., Servier, Lexicon Pharmaceuticals, Windtree Therapeutics, among others.
Acute Coronary Syndrome Market
Acute Coronary Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key ACS companies, including Novartis, Boehringer Idorsia Pharmaceuticals, Viatris, Recardio, AstraZeneca, Faraday Pharmaceuticals, DalCor Pharmaceuticals, Roche, Jiangsu Vcare PharmaTech, CeleCor Therapeutics, Novo Nordisk, Bristol Myers Squibb, Johnson & Johnson Innovative Medicine, CellProthera, BioCardia, Kancera, Amgen, Arrowhead Pharmaceuticals, Abcentra, among others.
Acute Coronary Syndrome Pipeline
Acute Coronary Syndrome Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key acute coronary syndrome companies, including Janssen Research & Development, LLC, DalCor Pharmaceuticals, Hyloris Pharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
